



















The conjugate acid of an entity is the same entity with one hydrogen added on. HNO3 is the conjugate acid of the nitrate ion. 4 0. courseault. Lv 4. 4 years ago. Conjugate Acid Of No3. Source(s): https://shrinke.im/a9yxK. 0 0. Still have questions? Get your answers by asking now. Ask Question + 100. Join Yahoo Answers and get 100 points today. Join. Trending Questions. Trending Questions. is ... well NH3 is a base that reacts with H2O to get NH4 + OH- NH3+ H2O-->NH4+ + OH- A conjugate base is the species formed when a Bronsted- Lowry base accepts a proton. NH4+ is the conjugate acid of NH3. conjugate acid-base pairs differ by only 1 H+. If NH3 is the base, then the conjugate acid is NH4+ The conjugate acid of ammonia is the ammonium ion, NH_4^+. The conjugate acid of any species, is the original species PLUS a proton, H^+. Both mass and charge are conserved. So add a H^+ unit to NH_3, and I gets NH_4^+, ammonium ion. Are both mass and charge conserved here? By the same procedure, if I remove H^+ from any species, I get the conjugate base. The conjugate acid for N H3 N H 3 is N H+ 4 N H 4 + since a conjugate acid contains 1 more hydrogen atom and 1 more + charge in its chemical formula. Become a member and unlock all Study Answers... When NH3 acts as a base, it will donate its lone pair to a proton H+ and form its conjugate acid NH4+ whereas when NH3 acts as an acid, it can give out H+ ion and forms a conjugate base as NH2-. Reactions are given below: (Acting as a Lewis Base) NH3 + H+ ——-> NH4+. (Acting as a Lewis Acid) NH3 ——–> NH2- + H+. The conjugate acid of NH3 is NH4+. As acid is one that donate H+ ions while Base is one that accept H+ ion. So NH3 as base accept H+ ion and convert itself into its conjugate acid NH4+. As acid is one that donate H+ ions while Base is one that accept H+ ion.
[index] [4824] [2336] [5201] [2461] [4999] [5934] [1912] [3646] [6537] [6706]
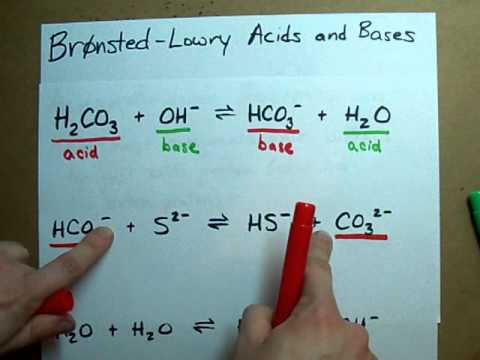
Hello this is a posting to an answer to a homework problem I have received. I am currently a student some university. The answers that are provided will be c... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... weak base, NH3, and its conjugate acid in the form of the salt, NH4Cl (ammonium chloride) Conjugate acids and bases are usually introduced in organ... Skip navigation Sign in. Search. Loading... Close. This video is unavailable. Watch Queue Queue. Watch Queue Queue. In order to balance NH3 + HCl = NH4Cl you'll need to be sure to count all of atoms on each side of the chemical equation. Once you know how many of each t... View full question and answer details: https://www.wyzant.com/resources/answers/706592/identify-the-following-reaction-label-the-acid-conjugate-acid-base-and... To make a carbonate buffer of pH 10.00, how many grams of sodium carbonate (Na2CO3) must you add to 1.5 L of freshly prepared 0.20 M sodium bicarbonate to ma... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺)....
Copyright © 2024 m.sportbetbonus772.casa